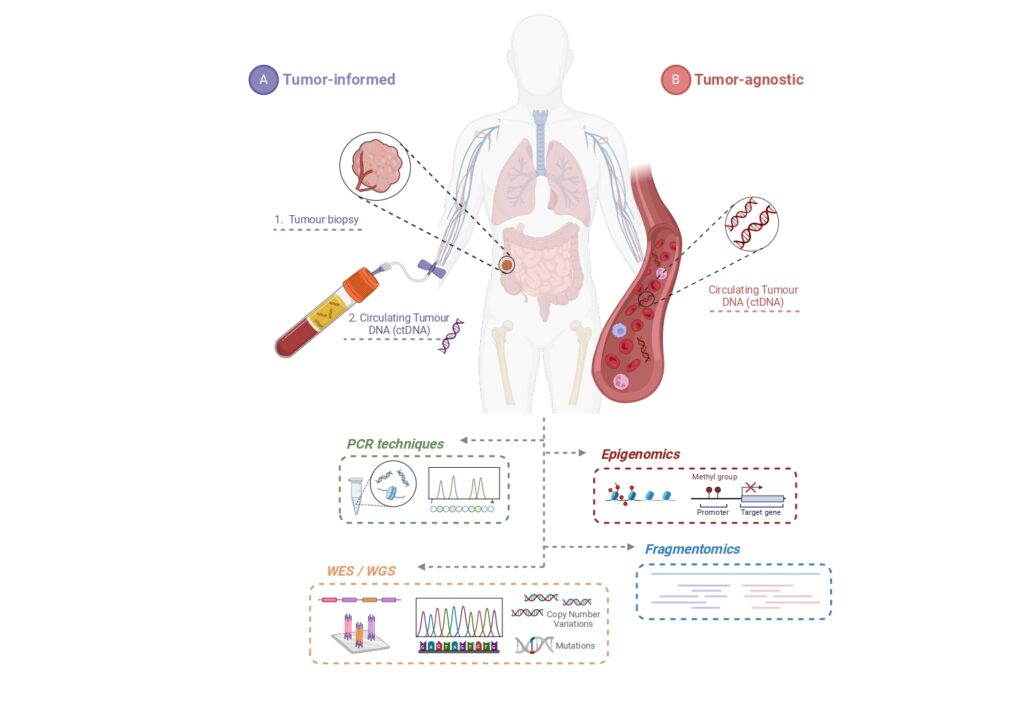
The significance of liquid biopsy through ctDNA detection has been reinforced in numerous cancers beyond colorectal cancer. Studies, including the DYNAMIC trial, highlight its potential in guiding treatment decisions within randomized clinical trials. However, this rapidly evolving field has sparked critical questions: What is the superior ctDNA detection method-tumor-informed or tumor-agnostic approach? Should clinical trials prioritize a “test-then-randomize” or “randomize-then-test” approach? Furthermore, while ctDNA clearance as a primary endpoint shows promise, recent findings suggest the need for refined definitions and methodologies.
A review in Annals of Oncology by Martínez-Castedo B, et al delves into these debates, comparing tumor-informed strategies that offer high sensitivity via personalized assays with tumor-agnostic approaches that prioritize accessibility but face sensitivity challenges. The integration of emerging technologies like fragmentomics and multi-omics holds the key to overcoming current limitations, paving the way for personalized and adaptive cancer care.
Stay tuned as ongoing trials and innovations promise to redefine the landscape of ctDNA analysis and its role in precision oncology. Read here: https://www.annalsofoncology.org/article/S0923-7534(24)04981-0/abstract
Written by Dr. Noelia Tarazona